I was inspired to write about the alkaline diet as I see more and more influencers who look phenomenal, attribute their health and looks to the pH of the foods they eat. There are on the other hand, those who follow an alkaline diet who look as though a gust of wind would cause them to fall and break a bone.
The foods promoted in the alkaline diet are of the likes of blueberries, spinach, kale, apples, banana and other foods generally known as healthy.
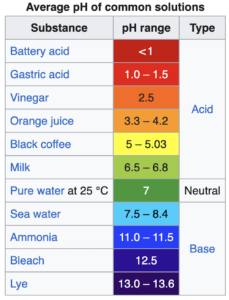
The pH scale runs from 1-14 with 1 being acidic, 7 neutral and 14 alkaline. pH stands for Power of Hydrogen. Hydrogen ions or protons are positively charged. They readily steal a pair of electrons from other substances added to its solution and corrode the substance. Acids break things down. That makes it sound like we might want to stay away from acids, doesn’t it!? It certainly does to me.
If you read my last article on homeostasis, our blood pH also has a homeostasis of around 7.4. It is pretty tightly regulated and cannot be easily influenced by our diet. This is for a reason, because it functions best in this narrow homeostatic range.
I’ve included a number of peer reviewed articles to back my claims but I’d like to start by first suggesting that we forget about getting overly ‘sciency’ for one minute and just use common sense. The foods listed in the alkaline diet are certainly contributing the strengthening of people’s health, but it’s not because of the pH of the foods. It is because those foods are nutrient dense functional foods.
Secondly, those foods are not even alkaline! You can test this yourself. You can get a pH meter, use distilled water with a pH of 7 which is neutral. Blend a food of choice with that water and test the pH with the meter.
You’ll be shocked to learn that the vast majority of foods are slightly acidic or neutral. Blueberries have a pH of 4. Green apples 3.5 due to the malic acid. Celery is a 7 pH. I’ll stop there to save on the length of this article. These are all part of the formal alkaline diet!
Here is some more common sense. You have stomach acid which breaks down your foods so nutrients can be absorbed. If you take something alkaline, like alkaline water or sodium bicarbonate and dump it into your stomach, you are neutralizing your stomach acid which in turn is going to hinder the absorption of nutrients from your food.
There is something called the alkaline response of foods. This is part of normal digestion of all foods including meat and junk food which turn into acid in the stomach. Then as the chyme leaves the stomach all the stomach and dietary acids are neutralized by the release of pancreatic bicarbonate (which is alkaline) from the pancreas to protect the intestines from burning.
This is the alkaline response which has no effect on blood pH or tissues in general. Virtually all pH in the body is regulated through two things; respiration and kidney function. Also note that a high urine pH does not mean your body is alkaline.
The only area that could go alkaline is between the kidneys and bladder. Alkaline bladders and vaginas are the perfect environment for yeast infections and UTIs. The vagina is supposed to have a pH of around 3.8-4.2 which is acidic, Not alkaline! Antibiotics can actually cause yeast infections by raising the pH of vagina. Male ejaculate however is alkaline so the sperm can survive.
Numerous studies (referenced below) show that the foods you eat do not have an effect on blood pH.
Here is more common sense… The body is made up of acids!
We cannot survive without acids. Lactic acid is a source of fuel for mitochondria.
The body regulates its pH and has various pH’s. There are parts of the body that have to be acidic. One of the reasons is to protect against pathogens. Stomach acid kills pathogens like H. pylori and Candida which thrive in alkaline environments.
Hyperventilation will cause a temporary alkaline state because you are blowing off carbon dioxide (carbonic acid) which raises your pH and you end up passing out. The CO2 helps maintain dilated blood vessels. When you are too alkaline the blood vessels constrict causing a decrease of blood flow to the brain, thus you pass out, causing your respiration to slow down or stop temporarily which builds the CO2 (acidity) back in the body.
Acidosis and alkalosis are rare conditions because the body maintains its pH. Being either too alkaline or acidic can actually kill you.
Urinary pH and salivary pH don’t reflect your blood pH. Even thinking about a certain food will change your salivary pH towards a more acidic state to get ready to break down and digest foods.
Parts of the body exposed to the outside world have to be acidic; skin, vagina, stomach, sinuses, otherwise when pathogenic bacteria land on your skin they’d have greater chances of proliferating.
The nutrients which are required for stomach acid; B6, b12 and folate all require acid to be absorbed. When you keep neutralizing your stomach acid you are impeding your body’s ability to produce stomach acid in the long run.
I hope this helps put the final nail in the coffin of the alkaline diet. Especially as it relates to our gut health. We already have so much to concern ourselves with when it comes to living a healthy life and maintaining these machines we call the human body. To focus on the alkalinity of foods is work that will not bear any fruit. Pun intended.